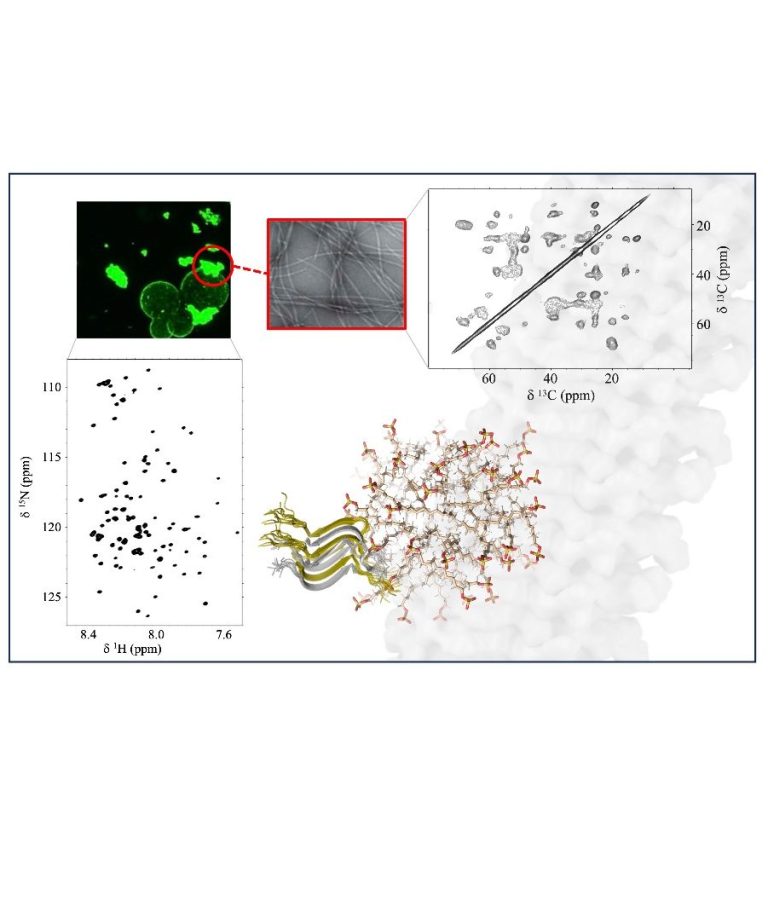
NMR of biosolids and supramolecular assembly
In the complex world of cellular function and human health challenges, the process of protein aggregation remains a fascinating puzzle. Our research is dedicated to uncovering the intricate molecular details behind a variety of supramolecular assemblies. We explore how proteins come together during liquid-liquid phase separation and homo- and heteromeric amyloid assembly, both in diluted phases and as biomolecular condensates shift from being liquid to solid. We are also delving into the influence of lipids in these processes. Our mission involves a comprehensive examination of the many factors at play, from the thermodynamics and kinetics to other key aspects governing the resulting conformations and dynamics of the systems. To answer these questions, we use an integrative approach that leverages cutting-edge NMR techniques, both in solution and in the solid-state, and complemented with hyperpolarization schemes and a diverse array of computational methods.
Our research encompasses a broad spectrum of health-related proteins, including but not limited to TDP-43, nucleoporins, RIP kinases, and other proteins containing RHIM domains. Ultimately, the insights gained from our investigations are channeled toward the design of new proteins with improved capabilities for biotech purposes, ranging from catalytic hubs to modulators of distinct aggregation events.