Self-assembly is a foundation of life, but what about co-assembly?
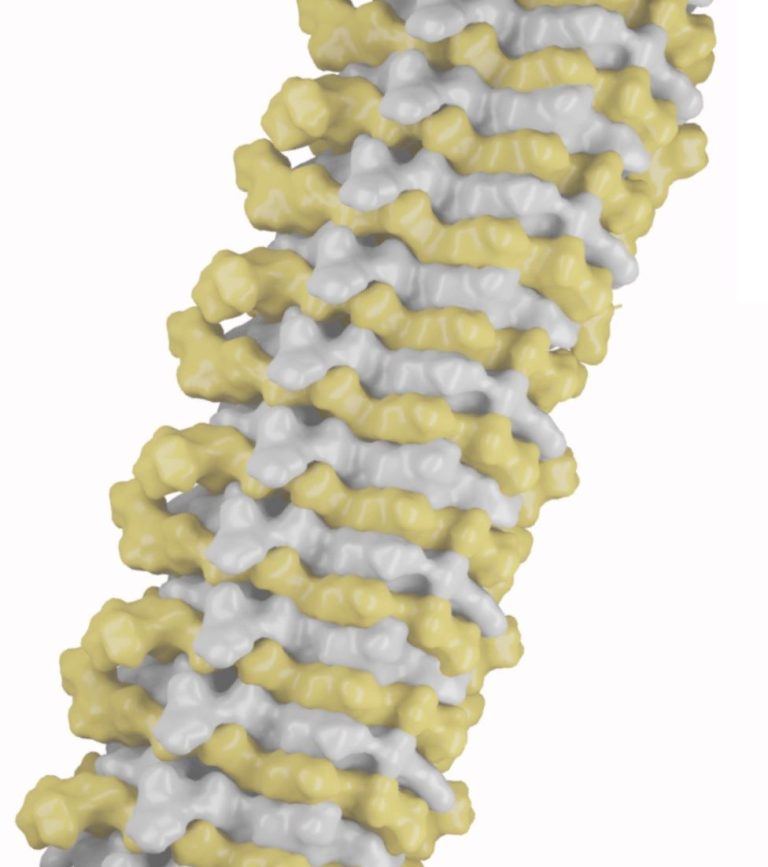
BiFOLDOME aims to decipher the molecular basis of amyloid co-assembly to understand self-assembly. Amyloids were assumed to be assembled by one type of protein, but the discovery of 1:1 co-assembled heteromeric amyloid fibrils suggests that amyloids composed of two distinct proteins playing key roles in health and disease may be common. In fact, certain viral proteins assemble heteromeric fibrils with host proteins to selectively hijack cell regulation. Taking a leaf from the viral playbook, this means that for a given self-assembling sequence there may be a mating sequence driving the preferential 1:1 co-assembly of the two. Thus, understanding what drives the preferential formation of co-assembled forms over conventional self-assembled species may provide an entirely new perspective on assembly processes that would cut across all fields of knowledge.